A Manufacturing and Quality Focused Approach In the disposable urinary catheter market, product quality is often judged by whether a sample “passes” initial evaluation. However, long-term cooperation depends on something more critical: manufacturing consistency and failure risk control. Based on industry experience, the most common non-clinical catheter failure issues kinking, leakage, and uneven hydrophilic coating are rarely caused by a single factor. They usually result from cumulative weaknesses in design decisions, process stability, and quality control. Below is how we systematically address these risks throughout product development and manufacturing. 1.Reducing Kinking Risk Through Balanced Structural Design Catheter flexibility is important, but excessive softness without structural support increases the risk of kinking and lumen collapse. Instead of pursuing softness alone, we focus on balanced mechanical performance. Our approach includes: Wall thickness design optimized to maintain lumen integrity under bending Material formulation selected to provide both flexibility and elastic recovery Stable extrusion processes to minimize wall thickness variation Internal checks to identify deformation risks during handling and packaging In addition, packaging methods are designed to reduce stress concentration and prevent permanent creases during storage and transportation. Outcome: Catheters that remain flexible while maintaining consistent lumen shape and flow reliability. 2.Preventing Leakage Through Process Control and Dimensional Consistency Leakage is one of the most frequent causes of customer complaints, yet it is rarely a random defect. In most cases, leakage originates from dimensional mismatch, bonding instability, or process variation. To reduce this risk, we emphasize: Tight dimensional matching between tubing and connectors Defined bonding or welding parameters with controlled tolerances Incoming material consistency monitoring to reduce batch variation In-process inspection combined with final leak integrity checks Rather than relying solely on end-of-line inspection, we focus on process repeatability, which plays a greater role in long-term quality stability. Outcome: Improved sealing reliability and reduced variability across production batches. 3.Ensuring Uniform Hydrophilic Coating Performance For hydrophilic-coated urinary catheters, the presence of a coating alone is not sufficient. Uniformity and durability are critical to consistent performance. Uneven coating thickness or insufficient curing can result in localized friction, unpredictable lubricity, or performance degradation over time. To address this, we focus on: Controlled coating application parameters Defined curing and crosslinking conditions Internal evaluation of coating consistency along the catheter surface Compatibility assessment between coating materials and packaging systems By treating coating performance as a system rather than a single step, we reduce hidden variability that may only appear during use. Outcome: More consistent surface lubricity and predictable handling characteristics. 4.Quality Control Focused on Consistency, Not Just Compliance Passing basic compliance checks is only the starting point. For long-term supply stability, repeatability and traceability are equally important. Our quality approach emphasizes: Multi-batch sample evaluation during project initiation Process monitoring instead of dependence on final inspection alone Batch traceability to support long-term quality review Clear communication during sample approval, pilot runs, and scale-up This allows potential risks to be identified earlier and addressed before volume production. Outcome: Greater confidence for brand owners and distributors in long-term cooperation. 5.Supporting Buyers Beyond the First Sample From a buyer's perspective, catheter quality is not defined by a single approved sample it is defined by how reliably that sample can be reproduced over time. We support this by offering: Sample comparison across different batches Small-batch pilot production before scale-up Technical communication during design and specification review Ongoing feedback integration during production lifecycle This approach helps reduce unexpected quality issues and minimizes the need for repeated requalification. Why This Approach Matters for Procurement and Brand Owners Uncontrolled failure risks often lead to hidden costs, including: Customer complaints and returns Shipment delays or rework Additional supplier audits or revalidation By addressing common failure modes at the design and manufacturing level, these risks can be significantly reduced, supporting more stable supply chains and long-term partnerships. Ready to Discuss Your Catheter Project? Whether you are sourcing standard disposable urinary catheters or developing hydrophilic-coated solutions, we support evaluation at every stage from samples to scalable production. Contact us to discuss your requirements or request samples.
-
Company News Dec 03,2025
As the global population ages, more seniors face daily urinary challenges, from incomplete bladder emptying to mobility limitations. For families providing home care, managing these needs becomes a key part of daily life. Catheters, especially intermittent ones, play a critical role in helping seniors maintain comfort, hygiene, and independence. At BEVER Medical, we believe a catheter is not just a medical device it is something that interacts with a person's most private daily routines. That's why our design philosophy starts with empathy: understanding the unique needs of seniors and caregivers to create products that are comfortable, safe, and easy to use. Understanding the Needs of Seniors Seniors often have weaker immunity, thinner skin, and reduced hand strength, making them more susceptible to infections or injuries during catheterization. Some rely on caregivers, while others attempt self-catheterization with limited mobility. Consider Mrs. Li, 78, who lives independently. Traditional catheters can be uncomfortable, and the process of using extra lubricant or opening complicated packaging adds frustration. Observing real-life experiences like hers guides every design decision. Key considerations for senior-friendly catheter design include: Safety and hygiene: Minimizing infection risk Comfort and minimal pain: Reducing friction during insertion Ease of use: Simple to handle, even with limited mobility Adaptability: Fit different anatomies and scenarios, from home care to travel Designing for Comfort and Safety Comfort begins with material. Catheters should use soft, flexible medical-grade polymers tailored for senior users. Every catheter tip should be rounded and polished to reduce friction and discomfort, helping seniors feel less anxious during insertion. Hydrophilic coatings are an important innovation in this area. Activated by water, these coatings create an ultra-smooth surface that minimizes friction and urethral irritation. For seniors, this means less pain, greater confidence, and a smoother experience, even with frequent catheter use. In addition, The Hydrophilic Coated Intermittent Catheters for senior users should bend enough to follow natural anatomy but remain firm enough for safe, controlled insertion. Every detail counts when comfort and safety are both top priorities. Practical Packaging for Home Care In home settings, sterility and convenience are critical. Packaging should be single-use, sterile, and easy to open, even for seniors with reduced hand strength. Clear instructions and intuitive layouts reduce errors and stress for both seniors and caregivers. While no catheter can fully eliminate infection risk, thoughtful packaging and design make daily care safer, simpler, and less stressful. Supporting Caregivers Many seniors depend on family members or professional caregivers. Catheterization can be physically and emotionally demanding. Features such as flexible shafts, intuitive packaging, and multiple sizes help caregivers perform care efficiently while maintaining the senior's dignity. Even small details: like color-coded tips or grip-friendly tabs can make a meaningful difference in daily routines, helping caregivers complete the process safely and with confidence. Empowering Independence The ultimate goal of senior-friendly catheter design is to support dignity and independence. A well-designed catheter reduces anxiety, improves confidence, and encourages seniors to follow recommended catheterization schedules. This not only improves bladder management but also enhances overall quality of life. Take Mr. Zhang, 82, who self-catheterizes three times a day. Using a smooth, easy-to-use catheter allows him to follow his routine with minimal discomfort, helping him maintain independence and reassuring his family. Our Commitment Designing senior-friendly catheters is an ongoing mission. BEVER Medical continuously integrates user feedback, clinical research, and material innovations to improve comfort, safety, and usability. Every improvement from coatings to ergonomics to packaging moves us closer to a world where urinary care at home is simpler, safer, and more dignified for aging adults.
-
Company News Nov 05,2025
Intermittent catheters have become a cornerstone of modern urology care, offering patients and healthcare providers an effective alternative to indwelling catheters. They are widely used in hospitals, long-term care facilities, and home healthcare settings, particularly for individuals with neurogenic bladder, spinal cord injuries, or benign prostatic hyperplasia. For B2B buyers, understanding market trends, clinical evidence, and supplier capabilities is crucial for informed procurement decisions. 1. Market Trends Driving Intermittent Catheter Demand The global intermittent catheter market has experienced steady growth due to an aging population and increasing prevalence of urological conditions. Hydrophilic and pre-lubricated catheters now account for the majority of new sales, reflecting a shift toward products that reduce friction and lower the risk of catheter-associated urinary tract infections (CAUTIs). Hospitals and home-care providers increasingly prioritize patient comfort and infection prevention. Suppliers that offer a range of catheter types, including straight, coude-tip, and ready-to-use sterile designs, are better positioned to meet diverse B2B buyer needs. Flexible supply chains and OEM customization options also play a key role in supporting bulk procurement and private-label partnerships. 2. Clinical Advantages of Hydrophilic Catheters Clinical studies support the superiority of hydrophilic catheters over standard PVC options. Research shows that hydrophilic coatings reduce urethral trauma and decrease the incidence of CAUTIs, especially in high-risk populations such as spinal cord injury patients. For healthcare providers, this evidence underscores the importance of selecting catheters that are both safe and effective for long-term use. Additional innovations, such as advanced catheter geometries that discourage bacterial migration, are emerging and may further improve patient outcomes. These developments demonstrate that even seemingly simple consumables like intermittent catheters are evolving rapidly in response to clinical needs. 3. Key Considerations for B2B Buyers When sourcing intermittent catheters, procurement teams should evaluate suppliers based on several critical factors: Design & Tip Type: Straight catheters are suitable for general use, while coude-tip designs help navigate anatomical challenges such as enlarged prostate. Ensure suppliers offer the full range of sizes and lengths for both male and female patients. Material & Coating: Hydrophilic or pre-lubricated options reduce friction and infection risk. Request clinical validation data for coatings and antimicrobial safety. Regulatory Compliance: Suppliers should meet ISO 13485 or equivalent quality standards and provide batch-level documentation, biocompatibility testing, and microbial safety reports. Supply Chain & Customization: Consistent lead times, flexible packaging, and private-label options are essential for large-scale procurement. OEM partners who can accommodate custom specifications make it easier to meet unique hospital or distributor requirements. Support & Training: Suppliers who provide educational materials and technical guidance help healthcare staff optimize catheter use, particularly in home-care settings. 4. Why Consider BEVER Medical as an OEM Partner For buyers looking for a reliable OEM partner, BEVER Medical offers extensive experience in manufacturing hydrophilic and pre-lubricated intermittent catheters. Their services include: Custom catheter designs tailored to male and female patients Bulk production and private-label packaging options Compliance with international quality standards (ISO 13485, CE, FDA) Support for documentation, regulatory submissions, and training Collaborating with an experienced OEM like BEVER Medical can simplify procurement, ensure consistent product quality, and allow healthcare organizations or distributors to focus on patient care rather than supply logistics. 5. Emerging Trends and Future Outlook The intermittent catheter market continues to evolve with a focus on patient-centric care, infection control, and operational efficiency. Key trends include: Advanced coatings and catheter geometries to minimize contamination Patient-friendly designs suitable for self-catheterization at home Sustainable materials and packaging to meet environmental expectations Regulatory updates emphasizing clinical validation and infection prevention B2B buyers who stay informed about these developments can select products that deliver both clinical effectiveness and operational value. Conclusion For hospitals, long-term care facilities, and home-health agencies, choosing the right intermittent catheter requires careful consideration of design, material, clinical evidence, and supplier reliability. Partnering with a trusted OEM supplier like BEVER Medical can help procurement teams access high-quality, customizable solutions while ensuring patient safety and operational efficiency. Next Step: Contact BEVER Medical to explore OEM collaboration and bulk procurement options.
-
Company News Oct 23,2025
BEVER Medical is excited to announce its participation in MEDICA 2025, the world's leading medical trade fair, taking place in Düsseldorf, Germany, from November 17–20, 2025. Visitors can find BEVER Medical at Booth 6L34-A, where the company will present its latest innovations in catheter and airway management solutions. About BEVER Medical As one of the world's leading high-quality medical device companies, BEVER Medical is dedicated to delivering safe, comfortable, and reliable products. With extensive expertise in intermittent catheters, airway management, and suction catheters, the company has earned the trust of healthcare providers and partners worldwide through continuous innovation and a strong commitment to quality. Featured Products at MEDICA 2025 Hydrophilic Catheters BEVER Medical will highlight its latest Hydrophilic catheters, designed for smooth, safe, and comfortable use. Nasopharyngeal Airways and Suction Catheters In addition, the company will showcase nasopharyngeal airways and suction catheters, ideal for emergency care, clinical procedures, and everyday hospital use. Each product reflects BEVER Medical's ongoing dedication to innovative design, patient experience, and clinical reliability. OEM and Tailored Solutions Beyond high-quality products, BEVER Medical offers comprehensive OEM services. From product design and testing to manufacturing and quality control, the company provides tailored solutions that help global partners bring safe, efficient, and cost-effective medical devices to market quickly while meeting rigorous standards. Engage with Our Team During the fair, visitors will have the opportunity to meet BEVER Medical's expert team, explore live product demonstrations, and learn more about the company's collaborative approach to healthcare innovation. BEVER Medical looks forward to discussing potential partnerships, product customization, and joint development opportunities with industry professionals. Connecting Globally at MEDICA 2025 MEDICA 2025 provides an ideal platform for BEVER Medical to connect with healthcare providers, distributors, and innovators from around the world. By showcasing its latest technologies and solutions, the company aims to contribute to the advancement of patient care, clinical efficiency, and medical safety on a global scale. Visit Us For more information about BEVER Medical's products and services, visit www.bevermedical.comOr stop by Booth 6L34-A to experience cutting-edge medical solutions and explore partnership opportunities.
-
News Aug 29,2025
Looking for OEM or bulk airway management devices? Global distributors and hospital procurement teams rely on endotracheal tubes (ETTs) and nasopharyngeal airways (NPAs) to ensure safe and efficient patient care. Sourcing through OEM or bulk supply channels provides cost savings, reliable inventory, and private-label branding opportunities. This guide explains how to choose the right supplier, highlights BEVER Medical's offerings, and outlines best practices for bulk purchasing. Why OEM and Bulk Supply Matter Bulk procurement and OEM partnerships offer several advantages for distributors and buyers: Cost Efficiency: Lower per-unit prices for large-volume orders Inventory Stability: Ensures continuous availability for hospitals and emergency services Brand Differentiation: Private-label options enhance market recognition Regulatory Compliance: ISO, CE, and FDA-certified manufacturers simplify international sales BEVER Medical A Trusted OEM Partner Founded in 2008, BEVER Medical manufactures medical devices in an ISO 8 Class 100,000 cleanroom of over 2,000 m². Their capabilities include: Advanced plastic extrusion and injection molding OEM customization: private-label branding, custom packaging, size variations Scalable bulk production for mid-sized and large-volume orders Regulatory support for global markets BEVER Medical is a reliable partner for distributors seeking high-quality airway devices with consistent supply. Product Focus Endotracheal Tubes (ETTs) ETTs are critical for airway management in anesthesia, ICUs, and emergency care. Key features for buyers: Tube Types & Sizes: Cuffed, uncuffed, reinforced, pediatric options Material Options: PVC, silicone, or hydrophilic-coated tubes Sterility & Packaging: Single-use sterile packaging minimizes infection risk OEM Customization: Private-labeling and tailored packaging Buying Tip: Partnering with an ISO-certified manufacturer ensures batch-to-batch consistency and regulatory compliance. Product Focus Nasopharyngeal Airway With Lubricant (NPA) NPAs maintain airway patency in emergency care or anesthesia. BEVER Medical's NPA features: Medical-Grade Material: Soft PVC reduces patient trauma Pre-Lubricated Design: Smooth insertion and improved comfort Multiple Sizes: Adults and pediatric patients Sterile, Single-Use Packaging: Prevents cross-contamination OEM Customization: Private-labeling for global distributors High-turnover product: Bulk supply is essential for hospitals, emergency services, and first responders. How to Choose an OEM Supplier Global buyers should consider these criteria: Regulatory Compliance: ISO 13485, CE, FDA Production Capacity: Cleanrooms, automated machinery, scalable output Customization: Private-label branding, packaging, material options Bulk Supply Flexibility: Minimum order quantities and lead times Logistics Support: Experience with international shipping and documentation A reliable supplier reduces risk, ensures product quality, and supports consistent market delivery. Advantages of OEM & Bulk Sourcing Cost Savings: Lower unit prices for high-volume orders Inventory Reliability: Consistent availability for clinical and emergency needs Brand Building: OEM options allow market differentiation Compliance Assurance: Certified manufacturers assist with local regulations Conclusion Endotracheal tubes and nasopharyngeal airways are vital in clinical airway management. For distributors, resellers, and hospital procurement teams, OEM and bulk supply strategies provide cost efficiency, inventory stability, and brand differentiation. Partnering with an experienced and certified manufacturer like BEVER Medical ensures reliable, high-quality devices while meeting growing global demand. Looking for an OEM partner for endotracheal tubes and nasopharyngeal airways? Contact BEVER Medical today to explore bulk supply and customized solutions tailored for global buyers.
-
Company News Jul 25,2025
In the global healthcare market, the demand for specialized urological devices is steadily increasing. Among these, the Coude catheter stands out as a critical solution for patients facing challenges with standard urinary catheterization, particularly those with anatomical complexities such as benign prostatic hyperplasia (BPH) or urethral strictures. For medical brands, distributors, and healthcare providers aiming to offer tailored solutions, OEM and private label coude catheters represent a strategic opportunity to deliver high-quality, customized products under their own brand. Understanding the Coude Catheter and Its Clinical Significance A Coude catheter is characterized by its uniquely angled or curved tip, designed specifically to facilitate catheterization in male patients who experience difficulty with traditional straight-tip catheters. The curved design allows the catheter to navigate around obstructions or anatomical curves within the urethra more effectively, reducing patient discomfort and the risk of trauma during insertion. Common clinical indications for using a Coude catheter include: Benign prostatic hyperplasia (BPH), which causes enlargement of the prostate gland and obstruction of urinary flow. Urethral strictures or narrowing, which can impede the passage of standard catheters. Patients who have experienced repeated failed catheterization attempts with straight catheters. This specialized design not only improves clinical outcomes but also enhances patient comfort and reduces procedural complications, making the Coude catheter an essential device in urology and long-term catheter management. The Value of OEM and Private Label Solutions for Coude Catheters With increasing market competition and a growing need for product differentiation, many healthcare companies and distributors turn to OEM (Original Equipment Manufacturer) and private label manufacturing as viable approaches to expand their product portfolios efficiently. These models offer several advantages: Cost Efficiency: OEM production eliminates the need for significant capital investment in manufacturing facilities and equipment. Speed to Market: Leveraging an experienced manufacturer’s existing production capabilities accelerates product launch timelines. Customization: Brands can tailor product specifications—including materials, sizes, coatings, and packaging—to meet the preferences of specific markets or clinical requirements. Regulatory Compliance: Partnering with an ISO-certified manufacturer ensures adherence to stringent quality standards and facilitates certification processes such as FDA 510(k) or CE marking. Brand Identity: Private labeling allows companies to market products under their own brand, enhancing customer loyalty and market positioning. Customization Capabilities to Meet Diverse Clinical and Market Needs A reputable OEM partner offers extensive customization options to suit varying clinical needs and market demands. When it comes to the Coude catheter, key customizable elements typically include: Tip Design: Variations such as the classic Coude tip, Tiemann tip, or olive tip to accommodate different anatomical challenges. Material Composition: Options range from medical-grade PVC and silicone to latex-free and DEHP-free materials, ensuring biocompatibility and patient safety. Surface Coatings: Hydrophilic coatings or pre-lubricated options reduce friction during insertion, enhancing patient comfort and lowering the risk of urethral injury. Size Range: Availability of a broad French size range (typically 6Fr to 24Fr) to meet pediatric, female, and male patient requirements. Packaging and Labeling: Custom sterile packaging designs, multi-language instructions for use (IFUs), barcodes, and compliance with Unique Device Identification (UDI) regulations. Certifications and Documentation: Support for regulatory submissions and quality audits with comprehensive technical files, biocompatibility reports, and sterilization validation. These capabilities ensure that OEM and private label coude catheters can be precisely adapted to meet the clinical needs of diverse patient populations as well as the regulatory demands of various international markets. Ensuring Quality and Regulatory Compliance Quality assurance is paramount in the manufacturing of medical devices. Trusted OEM manufacturers operate under internationally recognized quality management systems such as ISO 13485, ensuring consistent product quality and safety. Additionally, they provide robust documentation to support regulatory approvals including FDA 510(k) in the United States and CE marking for the European Economic Area. By partnering with an experienced OEM supplier, brands gain access to validated manufacturing processes, thorough quality control protocols, and regulatory expertise. This not only mitigates risk but also streamlines product registration and market access. Strategic Advantages of Partnering with a Professional OEM for Coude Catheters Collaborating with a seasoned OEM partner delivers numerous business benefits: Reduced Operational Complexity: Outsourcing manufacturing allows your team to focus on sales, marketing, and distribution. Access to Technical Expertise: OEM partners often offer design consultation, prototyping, and performance testing services. Scalable Production: Ability to meet variable order volumes from pilot batches to full-scale production. Global Market Reach: Manufacturers experienced in international trade support logistics, packaging compliance, and after-sales service. Brand Differentiation: Tailored product features and packaging help your brand stand out in a competitive market. Conclusion: Elevate Your Urology Product Line with OEM & Private Label Coude Catheters In an evolving healthcare landscape, the ability to provide specialized, high-quality urological devices is a competitive necessity. Leveraging OEM and private label manufacturing for Coude catheters empowers your brand to meet specific clinical needs, ensure regulatory compliance, and accelerate time to market with a trusted manufacturing partner. If you are interested in developing customized coude catheter products that align perfectly with your brand vision and market strategy, contact us today. We provide comprehensive OEM solutions, including product customization, regulatory support, and global logistics services—helping you deliver excellence in urological care. If you're interested in Coude catheter products, be sure to check out this article. How do I choose the right coude catheters ?
-
Company News Jul 23,2025
As global demand for efficient and patient-friendly urology solutions grows, healthcare providers and medical distributors alike are seeking safer, more reliable catheter options. In particular, intermittent urinary catheters play a critical role in both clinical and home care settings. BEVER Medical's Ready-To-Use Hydrophilic Intermittent Catheter for men offers a smarter solution for the medical supply chain — combining ease of use, superior patient comfort, and international compliance for streamlined B2B distribution. What Is a Ready-To-Use Hydrophilic Catheter? Unlike standard catheters that require water or external lubricant, a ready to use hydrophilic catheter comes pre-lubricated and packaged in sterile saline or water. BEVER Medical's version eliminates the need for prep time, making it ideal for busy hospitals, nursing homes, and self-catheterizing patients. Key Benefits for Healthcare Providers: Faster application, reduced prep time Lower infection risk due to fewer handling steps More consistent and hygienic procedures Key Advantages for Distributors: Growing demand in both clinical and home care markets Easy-to-stock, ready-to-ship sterile packaging Strong market appeal across urology and elder care sectors Superior Comfort and Safety with Hydrophilic Coating BEVER Medical's intermittent catheter features an advanced hydrophilic coating that creates a smooth, lubricated surface when activated. This reduces friction and trauma during insertion or removal — improving the patient experience and reducing complications. Ideal for: Post-operative catheterization Long-term or repeat users Patients with spinal cord injuries or urinary retention Crafted from medical-grade, DEHP-free and latex-free materials, BEVER Medical's catheters ensure both comfort and regulatory safety across regions. No-Touch Sleeve for Infection Control Infection prevention is critical in any care environment. BEVER Medical includes a blue no-touch insertion sleeve, allowing caregivers or users to insert the catheter without directly handling the tubing. Infection control advantages: Meets international clinical hygiene standards Reduces cross-contamination risks Increases safety in hospitals, care homes, and self-use scenarios Built for B2B Distribution and Clinical Success BEVER Medical's ready-to-use catheter is more than just a product — it's a solution tailored for medical supply chains. CE and FDA certified – Global compliance ready CH08–CH18 sizing – Fits most adult male patients Individually packaged & sterile – Hospital- and home-use friendly OEM / ODM branding available – Ideal for private label catheter brands Export-ready logistics support – Quick lead times and global delivery Partner with BEVER – Your Trusted Intermittent Catheter Supplier Whether you're a regional catheter distributor, a hospital system buyer, or a healthcare brand expanding into urology medical devices, BEVER Medical is your ideal B2B partner. We provide scalable, high-quality, and ready-to-ship intermittent catheter solutions that deliver value at every level of the supply chain. Contact Us for Samples, OEM Pricing, or Distributorship
-
Company News Jun 20,2025
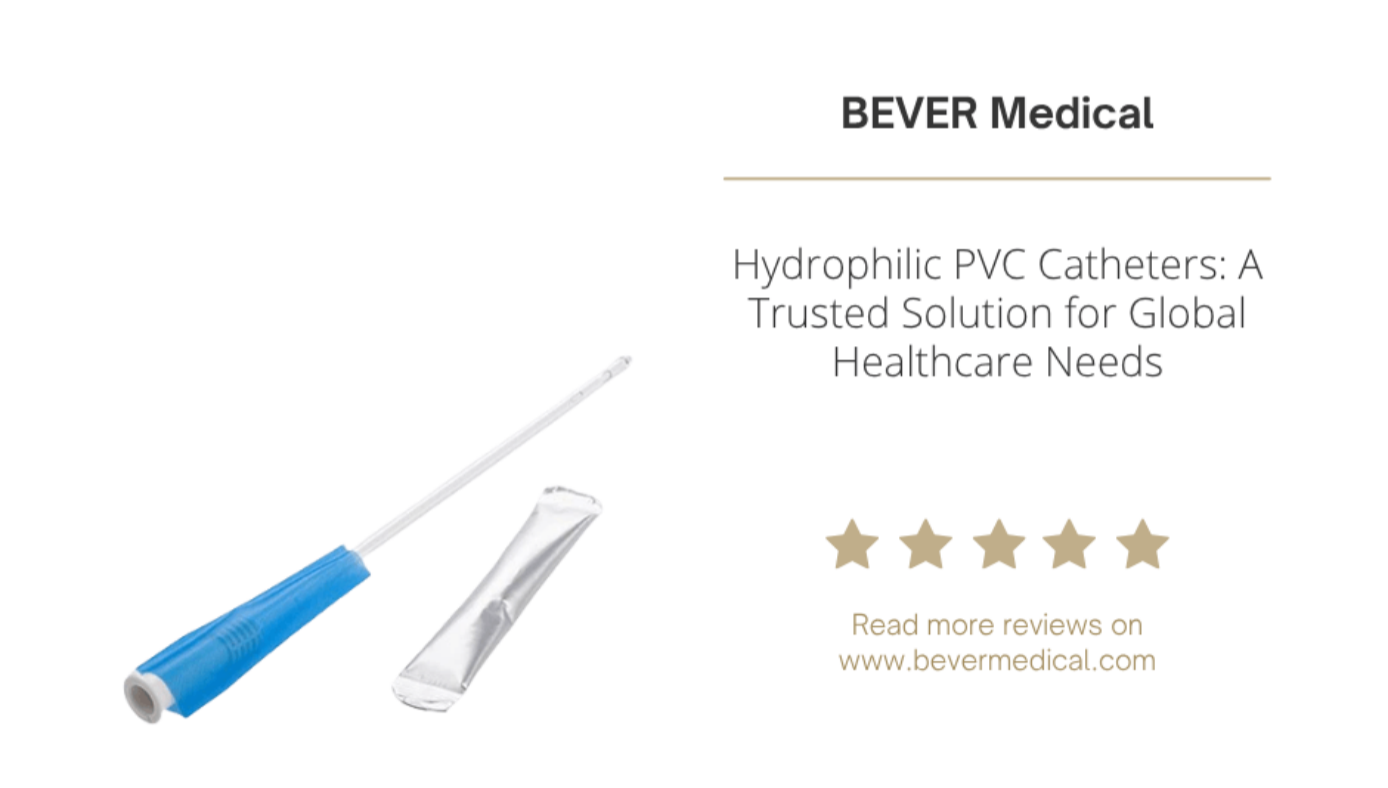
As the global healthcare industry focuses more on patient comfort and infection prevention, hydrophilic intermittent catheters have become a leading solution in urinary care. With their low-friction surface, sterile packaging, and ease of use, these catheters are rapidly replacing traditional uncoated options in both clinical and homecare settings. For procurement managers, urology brands, and medical distributors, selecting the right hydrophilic catheter is not just about product quality—it's about finding a supplier that delivers scalability, regulatory compliance, and private label support. What Is a Hydrophilic Catheter and Why Does It Matter? A hydrophilic catheter features a polymer coating that becomes ultra-smooth when activated by water. This reduces insertion resistance, minimizes urethral trauma, and improves patient comfort—particularly for individuals who require frequent self-catheterization, such as those with neurogenic bladder or post-surgical needs. Compared to manually lubricated catheters, hydrophilic versions offer: Improved sterility and hygiene Simplified use, especially for elderly or limited-mobility patients Lower risk of catheter-associated urinary tract infections (CAUTIs) Why Medical-Grade PVC Is the Material of Choice While silicone is often used in indwelling catheters, PVC (Polyvinyl Chloride) remains the top choice for intermittent use due to its balance of performance and price. Our hydrophilic catheters use DEHP-free, latex-free medical-grade PVC, offering several B2B advantages: Benefits of PVC Hydrophilic Catheters: Optimal stiffness for easy insertion without compromising comfort Superior coating compatibility for even water activation High-volume manufacturability for scalable global distribution Cost efficiency that aligns with tender requirements and insurance budgets Product Features Tailored for B2B Needs Our sterile, single-use hydrophilic PVC catheters are developed to serve hospitals, pharmacy chains, private healthcare brands, and humanitarian aid programs. Key Features: Water-activated hydrophilic coating or pre-lubricated option Available in straight tip and Coude tip for anatomical flexibility Color-coded funnel connectors (8Fr to 18Fr) for easy identification Rounded eyelets for reduced tissue trauma EO sterilized, individually packaged Bulk packaging or shelf-ready kits for various use environments Whether you serve acute care hospitals or homecare patients, our products deliver consistent performance and regulatory compliance. OEM & Private Label Catheter Solutions As both a manufacturer and a brand owner, we understand the flexibility and speed that international partners require. Our full-service OEM and private label programs are built to support: What We Offer: Custom branding and packaging design Multilingual IFUs and compliance labeling Flexible kit components (catheter, lubricant, gloves, urine bag) CE MDR & FDA 510(k) support, plus ISO 13485 certification Low minimum order quantities and short lead times We're already serving clients across Europe, Latin America, the Middle East, and Southeast Asia—with documentation and logistics support to match each region's needs. Why Distributors and Healthcare Buyers Trust Us Choosing the right hydrophilic catheter supplier isn't just about product specs—it's about partnership. At BEVER Medical, we provide: Stable, high-quality production capacity Regulatory and market access assistance Customizable solutions for multiple price points Fast sample turnaround and competitive global shipping Whether you're bidding for a government tender, launching a new retail brand, or expanding your urology catalog, we're here to help you grow—confidently and compliantly. Conclusion: A Smarter Choice for Global Urology Hydrophilic PVC catheters offer the perfect balance of clinical comfort, cost control, and compliance—backed by scalable manufacturing and customizable OEM services. As the demand for self-catheterization and sterile urology solutions grows worldwide, choosing a flexible, proven supplier becomes more important than ever. → Contact us today to request samples or discuss your private label needs.Let's create catheter solutions that fit your market—and your patients.
-
Company News Jun 17,2025
June 11–13, 2025 | Miami Beach Convention Center | Booth W45 Three days, thousands of professionals, countless meaningful connections — BEVER Medical's journey at the World Health Expo 2025 in Miami has been nothing short of extraordinary. We're proud to have stood alongside the world's leading innovators, showcasing how our patient-centric solutions in urology and respiratory care are helping reshape the global standard of care. What We Brought to WHX 2025 At Booth W45, visitors experienced firsthand the technologies driving our mission: Next-Generation Intermittent Catheters Hydrophilic Coating: Instantly lubricated for smooth, comfortable insertion No-Touch Design: Sterile, hygienic, and ideal for self-catheterization High-Quality PVC: DEHP-free, safe, and engineered for clinical reliability Nasopharyngeal Airway Solutions Standard, Lubricated, and Adjustable Options Silicone and PVC selections designed to reduce trauma and enhance patient safety Full size range (14Fr–38Fr) to meet emergency and long-term airway needs Advanced Suction Catheters Crafted for maximum suction performance and tissue protection Available in transparent or frosted finishes with dual eyelets and safety flanges A Space for Global Dialogue WHX Miami wasn't just an expo — it was a stage for dialogue, innovation, and new possibilities. We met physicians seeking better tools for patient comfort, distributors looking for trusted OEM partners, and hospital leaders in search of reliable, regulatory-compliant supply chains. These interactions strengthened our belief that true healthcare innovation begins with listening — and moves forward through collaboration. What's Next? Whether you visited our booth, joined our live demos, or simply picked up a catalog — thank you for making our time at WHX unforgettable. If you didn't get a chance to connect, let's keep the conversation going:[email protected] We're actively expanding our global partnerships and welcome new opportunities to deliver high-quality, accessible medical solutions worldwide. Together, we move forward — for patients, for professionals, and for better care.See you at the next global stage. — The BEVER Medical Commercial Team
-
Company News Jun 15,2025
He was there to steady your bike, check your fever at night, and stand quietly in the background of every milestone — just in case you needed him.Fathers don't always say “I love you” with words. They say it through actions — with protection, hard work, and quiet care. This Father's Day, it's our turn to give back. At BEVER Medical, we believe honoring Dad goes beyond gifts. It means safeguarding his health, his dignity, and his independence — especially as age brings new challenges. Care That Respects His Strength Many men silently manage urological conditions such as urinary retention, prostate recovery, or neurogenic bladder. These affect not only physical health, but also comfort and confidence. Our intermittent catheters are designed to help. From hydrophilic-coated options for smooth, comfortable insertion to uncoated types for customized care, our products are travel-ready, discreet, and user-friendly. They help reduce infection risk, support daily routines, and let fathers stay active and in control — just the way they've always preferred it. Every Small Action Matters Helping a parent with medical needs may feel new — but it's also a quiet act of love. Consider these simple ways to support him: Check his routine: Is he using the right size or style? Is it still working well for him? Restock in time: Avoid the stress of running out — make sure his supplies are ready. Talk about it: Honest conversations reduce stigma and show him he's not alone. Thank You, Dad To the fathers who worked tirelessly behind the scenes — thank you.Your steady presence gave us the strength to grow. Now we honor you by helping you stay strong, healthy, and cared for. This Father's Day, caring is the most powerful gift.From all of us at BEVER Medical— Happy Father's Day.
-
Company News May 30,2025
In emergency and critical care settings, maintaining a clear airway can mean the difference between life and death. Among the most reliable and non-invasive airway adjuncts available is the nasopharyngeal airway (NPA)—a soft tube designed to bypass obstructions and provide a clear path for airflow through the nasal passage. For B2B buyers, including hospital procurement teams, medical distributors, and OEM partners, selecting the right NPA product is not only a clinical decision but also a strategic one. It involves balancing quality, safety, availability, compliance, and cost-effectiveness. What Makes a High-Quality NPA for Institutional Use? When sourcing NPAs in volume, especially for emergency rooms, ambulance fleets, and ICU departments, there are several critical features to look for: Variety of Sizes: A well-rounded product line should offer a full range (typically 6.0–9.0 mm) to suit both adult and pediatric patients. Comfortable Materials: Soft, medical-grade PVC minimizes nasal trauma and enhances patient tolerance. Secure Design: A flared flange prevents over-insertion, while a beveled tip eases application. Sterile Packaging: Each unit should be individually packaged in sterile, easy-to-open pouches—ideal for fast-paced clinical environments. Latex-Free Composition: Essential for reducing allergic risks, particularly in sensitive populations. Why B2B Buyers Choose Trusted NPA Manufacturers Beyond product features, successful procurement hinges on finding a reliable manufacturer who understands B2B dynamics. That includes: OEM & Private Label Options: Tailored branding helps distributors and healthcare systems deliver consistent identity and marketing power. Regulatory Readiness: Look for ISO 13485-certified facilities and products with CE marking or FDA registration (depending on the market). Flexible Supply Capabilities: Global buyers value fast lead times, low minimum order quantities (MOQs), and efficient logistics support. Technical Documentation: From MSDS to sterilization reports, a professional supplier should readily provide documentation for compliance and tenders. Partner with Confidence At BEVER Medical, we help B2B clients around the world source high-quality nasopharyngeal airways designed for clinical reliability and procurement efficiency. Whether you're supplying to a national EMS program, equipping field hospitals, or expanding your medical product line, our team is ready to support your success. Request samples, download our product catalog, or contact our sales team for a personalized quotation. [email protected]
-
Company News May 19,2025
Miami Beach, FL — June 11-13, 2025 — BEVER Medical is glad to announce its participation in the World Health Expo 2025 (WHX Miami), the largest medical exhibition in America, taking place at the Miami Beach Convention Center from June 11–13, 2025. Organized by the American International Exchange Group and supported by the U.S. Medical Device Industry Association, this prestigious annual event draws thousands of healthcare professionals, distributors, investors, and executives from around the globe. Welcome to visit BEVER Medical at Booth W45 to explore our latest line of high-performance catheters, nasopharyngeal airways, and suction catheters — all designed to enhance patient safety, comfort, and ease of use. Event Details Exhibition Name: World Health Expo 2025 Date: June 11–13, 2025 Location: Miami Beach Convention Center, Florida, USA Organized by: American International Exchange Group Bever Medical Booth: W45 Advancing Catheter Technology for Patient Comfort BEVER Medical's catheter lineup offers cutting-edge features tailored to patient needs: Hydrophilic Coating Catheter: With a special hydrophilic layer activated immediately upon contact with water, the catheter becomes incredibly slippery, reducing friction and providing a virtually painless insertion experience. High-Quality PVC Catheters: Made from DEHP-free PVC with optimal hardness for balanced flexibility, these catheters feature bullet-shaped tips for smoother insertion and staggered, polished eyelets for improved urinary flow. No-Touch Sleeve Design: Our female catheter includes a no-touch sleeve, allowing for sterile insertion and removal without direct hand contact, effectively minimizing the risk of bacterial infection. Nasopharyngeal Airway Innovations BEVER Medical offers a full range of nasopharyngeal airway solutions: Standard Nasopharyngeal Airway: Latex-free, sterile, and individually packaged with a rounded, beveled tip for safer insertion and enhanced patient comfort. Lubricated Nasopharyngeal Airway: Comes with an attached surgical lubricant pack for quick application and is suitable for semi-conscious patients with an intact gag reflex. Available in a full size range (14Fr to 38Fr). Adjustable Nasopharyngeal Airway: Featuring a movable flange for personalized fit, this premium silicone airway reduces mucosal trauma and is ideal for repeated or long-term use. Advanced Suction Catheters Our suction catheter is crafted from non-toxic, DEHP-free medical PVC, designed with dual micro-holes and a flange to prevent tissue damage during suction. Available in frosted or transparent finishes, it's a reliable solution for clinical environments prioritizing safety and visibility. Why Attend WHX Expo? WHX Miami is the ultimate platform for BEVER Medical to present its medical innovations to a global audience. This year's theme focuses on AI in medical supply chains, revolutionary healthcare technologies, and industry-wide collaboration. With hundreds of top-tier exhibitors and thousands of industry professionals expected, it's a must-attend event for anyone shaping the future of healthcare. As a company committed to quality, safety, and continuous innovation, Bever Medical is excited to join the conversation and showcase our latest solutions that improve patient outcomes and streamline clinical operations. Join Us at Booth W45 We warmly invite all healthcare professionals, distributors, and business partners attending WHX Miami to stop by Booth W45. Meet our team, explore our product range, and discover how BEVER Medical can support your medical supply needs with innovative, high-quality solutions.
Interested in our products? Fill out the form and we’ll reply within one business day.



 English
English