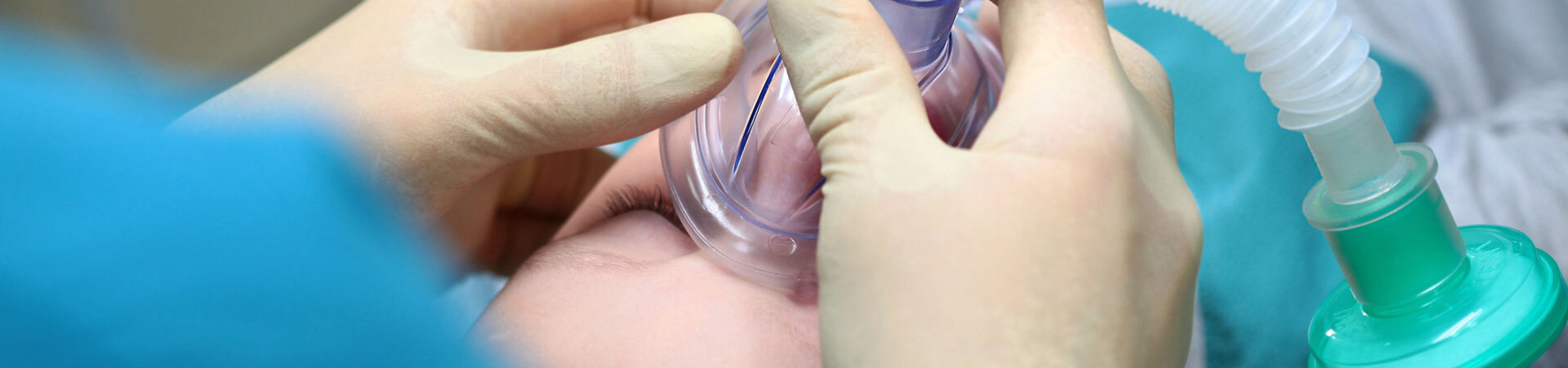
Nasopharyngeal airways (NPAs), commonly referred to as nasal trumpets, are indispensable tools in emergency airway management. From maintaining ventilation in unconscious patients to facilitating oxygenation during seizures or difficult intubations, NPAs serve vital roles across clinical and prehospital settings. A key decision facing medical professionals is whether to utilize reusable or disposable NPAs—a choice that impacts cost-efficiency, infection control, and operational readiness. This article compares both types, and shares some useful information to help you make the right choice. What Is a Nasopharyngeal Airway? A nasopharyngeal airway is a soft, flexible tube inserted into the nasal passage to secure an open airway. It bypasses obstructions at the tongue or soft palate level and is commonly used in: Pre-hospital emergency care Military combat casualty care Hospital ER or anesthesia First responder kits Depending on the material and design, NPAs fall into two categories: reusable and disposable. Types of Nasopharyngeal Airways Reusable NPAs Materials: Medical-grade silicone or rubber Key Traits: Soft, flexible, autoclavable Cleaning: Requires proper disinfection between uses Best for: Military, outdoor EMS, reusable kits Disposable NPAs Materials: PVC or thermoplastic elastomer (TPE) Key Traits: Pre-lubricated, sterile-packed, single-use No cleaning required Best for: ER, trauma bays, infectious environments Core Comparison: Reusable vs Disposable Criteria Reusable NPA Disposable NPA Cost per unit Higher upfront Lower upfront Long-term cost Cost-effective with repeated use Higher if used frequently Infection risk Requires strict disinfection protocol Minimal (single-use) Material Silicone or rubber PVC or TPE Durability High Moderate (meant for one-time use) Convenience Requires cleaning Ready-to-use Ideal users Tactical teams, field medics Hospital ER staff, paramedics Infection Control & Reusability One of the biggest differences between reusable and disposable NPAs is infection control. Reusable versions must be cleaned, disinfected, and stored properly. This introduces room for error, especially in high-pressure settings. Disposable NPAs eliminate this concern by ensuring a new sterile device for every patient. If you operate in high-infection-risk areas, like hospitals or disaster zones, a PVC nasopharyngeal airway is safer and more practical. Durability and Use Cases Reusable NPAs are designed to withstand harsh field conditions. They're often used in combat medical kits, wilderness EMS bags, and reusable nasopharyngeal airway kits. These devices bend without kinking and hold up well in extreme temperatures. By contrast, disposable NPAs are great for controlled environments where speed and sterility matter most, like: Emergency rooms Ambulance trauma response Anesthesia prep Scenario-Based Recommendation Hospital Emergency Rooms Disposable NPAs are the preferred choice. They save time, reduce cross-contamination risk, and eliminate cleaning tasks. Military and Outdoor EMS Reusable NPAs are ideal. They are durable, cost-effective over time, and can be packed with tactical supplies. Some versions are labeled combat-ready NPAs due to their rugged design. What Do the Experts Prefer? Anesthesiologists prefer disposables for speed and sterility.Combat medics often carry reusables for long missions or supply-limited zones.Ultimately, preferences depend on workflow, logistics, and safety protocol. Conclusion Choosing between reusable and disposable nasopharyngeal airways depends on your environment, frequency of use, and infection control needs. Disposable NPAs offer speed and sterility; reusable ones provide long-term savings and field reliability. If you don't know how to choose, asking the supplier may be a good choice. Importantly, follow the suggestions from you. Bever Medical offers a wide range of nasopharyngeal airways, including nasal trumpet, PVC Nasopharyngeal Airway, and full nasopharyngeal airway kit options tailored for hospitals, EMS, and combat-ready teams. Get in touch today to request a quote or sample!
View More +-
21 May 2025
In emergency and critical care settings, maintaining an open airway is non-negotiable. The nasopharyngeal airway (NPA) remains a trusted tool among clinicians for its ease of use, effectiveness in semi-conscious patients, and minimal invasiveness. Whether you're a hospital buyer, distributor, or tender manager, selecting the right NPA goes far beyond pricing—it's about safety, usability, and regulatory readiness. Reference factors for selection Here's what your facility should evaluate before making a purchase decision. 1. Material Selection: Safety and Comfort Start Here The material used in nasopharyngeal airways directly affects both patient comfort and clinical safety. Medical-Grade PVC: A widely used material, PVC offers a balance of cost and functionality. Leading brands now offer DEHP-free PVC, which eliminates exposure to harmful phthalates. Medical Silicone: A premium option, silicone is soft, flexible, and hypoallergenic—ideal for long-term or sensitive use cases such as pediatrics, ENT, or burn units. Ensure that whichever material you choose is latex-free to prevent allergic reactions, especially in regions where latex-free policies are mandated. 2. Size Range: One Device Doesn't Fit All A versatile NPA offering must accommodate all patient types—from neonates to large adults. The size of an NPA is typically indicated in French units (Fr), and a comprehensive range typically includes: 12Fr to 18Fr: For pediatric or neonatal patients 20Fr to 28Fr: For average adolescents and adults 30Fr to 36Fr: For larger adult patients or military/trauma scenarios Procurement teams should verify that suppliers offer clearly color-coded flanges corresponding to each size. This allows for quick visual identification, particularly useful during high-pressure emergencies. 3. Functional Design: Details That Drive Clinical Confidence An effective NPA should be designed to support safe, smooth insertion with minimal trauma. Look for: A beveled tip to ease insertion through the nasal passage A flexible body that adjusts to anatomy without collapsing A reinforced or wide flange to prevent migration into the nasal cavity Rounded or smoothed edges to reduce mucosal injury Clinicians often prefer NPAs with pre-lubrication or compatibility with water-based lubricants, which speeds up use and improves patient tolerance. 4. Sterility & Packaging: Compliance Begins Here Choose NPAs that are EO (ethylene oxide) sterilized and come in individual, tamper-proof packaging. Packaging formats may include: PE pouches (cost-efficient and compact) Blister packs (ideal for shelf visibility and protection) Ensure the label includes key information such as size, sterilization method, expiration date, and batch number. If your business serves international markets, look for packaging that supports multi-language Instructions for Use (IFU)—including Arabic, Portuguese, French, or Spanish. 5. Global Compliance: Not Optional in Today's Market For use in regulated markets such as the U.S., EU, Middle East, and Brazil, an NPA must meet certain certifications and documentation standards: ISO 13485:2016 for quality management systems CE certification under the EU MDR FDA device listing and registration (U.S. market) Documentation like Declaration of Conformity, sterility validation, and material safety reports Working with a compliant supplier helps you avoid delays in tenders and customs clearance, while reducing legal and clinical risks. 6. Distribution and OEM Flexibility: Empowering Your Brand For distributors, dealers, and private-label clients, flexibility in product delivery is a game-changer. A good supplier should offer: Low minimum order quantities (MOQs) Custom packaging and labeling options Mixed-size kits or full sets (ideal for EMS and emergency tenders) Quick lead times and support for international logistics If your brand is bidding on government projects or expanding into new markets, working with a flexible manufacturer will enable faster market entry and lower overhead. Why Choose BEVER Medical Nasopharyngeal Airways? At BEVER Medical, we understand that airway management is a high-stakes decision. That's why we've engineered our NPAs with global users in mind: Full size range: 12Fr to 36Fr, color-coded for clarity DEHP-free PVC or medical-grade silicone options Smooth beveled tips and flexible shafts for comfort EO-sterilized and individually packaged (PE or blister) ISO 13485 certified, CE MDR marked, FDA-listed OEM-ready with multi-language IFUs and low MOQs From pediatric hospitals in Brazil to military procurement in the Middle East and ambulance fleets in the U.S., BEVER Medical is a trusted partner in airway management. Ready to Simplify Sourcing? Let's Talk. Contact us today to: Request free samples Download our product catalog Get a custom OEM quote Access full compliance documentation for tenders
View More + -
29 May 2025
Nasopharyngeal airways (NPAs) help keep the nasal passages open in semi-conscious or unconscious patients, and are commonly used in emergency medical services (EMS), military settings, and hospitals. That's why distributors must source products that meet strict safety and compliance standards. Because NPAs are critical medical devices, sourcing them comes with unique challenges. With demand steadily rising, issues like shipping delays, incomplete size ranges, or product quality concerns can directly impact a distributor's business—and more importantly, patient safety. In this article, we'll walk through the most common sourcing problems and how to overcome them. Common Challenges in Sourcing NPAs 1. Delayed Delivery Slow lead times cause stockouts and missed opportunities. In emergency care, this can be disastrous. Distributors need a partner who understands urgency. 2. Incomplete Size Availability An effective airway solution must include a full size range, from pediatric to adult (14Fr to 38Fr). Gaps in inventory leave customers with unusable stock. 3. Poor Packaging Standards Unprofessional packaging damages credibility. Whether selling to EMS or a military unit, products must be sterile, sealed, and clearly labeled. 4. Quality and Material Complaints Distributors report issues with NPAs made from poor-quality PVC or rubber, leading to discomfort, breakage, or lack of biocompatibility. Choosing a Reliable Nasopharyngeal Airway Supplier Not all manufacturers are the same. When choosing a supplier, look for: Strict Quality Control: At Bever Medical, we use rigorous inspection systems at every production stage. Fast Shipping: We support rapid delivery for bulk and small orders. OEM Capability: Our custom packaging and branding options help your business stand out. Small MOQs: Ideal for new markets or regional testing. We help distributors reduce risks and grow confidently. Choosing FDA, CE, and Material Standards Regulatory compliance is essential. Always confirm: FDA Registration for U.S. market entry CE Marking for European distribution Use of medical-grade materials like PVC or silicone Products are latex-free, sterile, and single-use At Bever Medical, our PVC Nasopharyngeal airway and silicone models meet these requirements. We offer both lubricated and non-lubricated versions with features like beveled tips, round flanges, and ID marking. Supply Chain Management for NPAs Distributors should ask: Can the supplier support stable inventory? Are lead times realistic? Is there tracking and order visibility? Are they prepared for emergency resupply? Bever Medical ensures stock availability with warehousing options, consistent batch production, and export-ready logistics services—even in high-demand regions like nasopharyngeal airway army deployments. Cost Optimization Without Quality Sacrifice Price matters—but so does performance. To manage costs: Negotiate based on volume and delivery schedules Ask about OEM options for better margins Balance low MOQs with full-size range availability Choose suppliers who optimize freight with compact packaging We offer competitive pricing without compromising product safety or comfort. Final Thoughts Distributors play a vital role in connecting frontline responders with essential tools like nasopharyngeal airways. Whether you're supplying a local EMS team or stocking up for military field hospitals, having a dependable partner matters. Ready to streamline your NPA sourcing? Partner with Bever Medical for fast delivery, full-size options, and OEM support. Contact us today to learn more about our nasal trumpets, PVC Nasopharyngeal Airway, and adjustable models designed for EMS and nasopharyngeal airway army needs.
View More + -
19 Jun 2025
The nasopharyngeal airway (NPA) is a simple yet essential device used to secure the upper airway in unconscious or semi-conscious patients. Whether deployed in pre-hospital care, emergency rooms, or operating theaters, the NPA's effectiveness is closely tied to both its design features and material composition. For healthcare providers, procurement teams, and medical distributors, choosing the right NPA requires a careful evaluation of clinical functionality, patient safety, and cost efficiency. Key Design Features to Consider When selecting an NPA, look beyond basic sizing. The following elements significantly influence performance and usability: Size and Color Coding:Standardized color coding helps clinicians quickly identify appropriate sizes, especially in high-stress environments. Many NPAs also include length markings and clear labeling for traceability. Balance of Flexibility and Rigidity:An effective NPA must strike a balance—flexible enough to prevent trauma during insertion, yet rigid enough to prevent kinking or collapse inside the nasal passage. Safety Features:Modern NPAs typically incorporate a flared end, stopper ring, or external flange to prevent over-insertion. These design elements enhance safety, especially when used by first responders or in pediatric care. Material Comparison: PVC vs. Silicone Material selection is critical in ensuring the comfort, safety, and clinical appropriateness of the NPA. The two most common materials—PVC (polyvinyl chloride) and medical-grade silicone—each have distinct advantages based on the setting and user needs. PVC (Polyvinyl Chloride): Lightweight and firm, making insertion quick and intuitive Cost-effective for high-volume, single-use distribution Ideal for temporary use in low-stress environments such as field trauma kits or mass-casualty preparedness However, PVC is inherently less flexible and may cause discomfort or mucosal irritation if used without care, especially in smaller or pediatric patients. Silicone: Soft, flexible, and biocompatible, minimizing tissue trauma Maintains shape and performance in high-temperature environments, making it ideal for sterilized settings Suitable for prolonged use or patients with sensitive anatomy, such as children, elderly individuals, or surgical patients Silicone NPAs are more expensive but provide a higher degree of patient comfort and are often the preferred choice in ICUs, ORs, and pediatric units where quality of care is prioritized. Summary Table: Feature PVC Silicone Rigidity High (easier insertion) Moderate (gentler on tissue) Comfort Moderate Excellent Cost Low Higher Heat Resistance Limited Excellent Sterilization Disposable, EO only Autoclavable Biocompatibility Acceptable ISO 10993 certified Flexibility Lower High How Manufacturers Ensure NPA Quality When selecting an NPA supplier, it's crucial to consider how the product is manufactured and tested. Quality NPAs undergo comprehensive evaluations that include: 1.Material Certification (ISO 10993): Ensures the device is safe for mucosal contact, reducing risk of irritation or allergic response. 2.Insertion Force & Lubricant Compatibility Tests: Simulates real-use scenarios to ensure smooth, kink-free performance across various patient anatomies. 3.Sterilization and Packaging Standards: Individually packed and sterilized NPAs prevent contamination and support compliance with FDA, CE, and MDR regulations. Clear lot and batch markings enable full traceability for audits or recalls. Why NPAs Are a Must-Have in Emergency and Clinical Kits The NPA's role in airway management is irreplaceable in certain contexts—especially when oropharyngeal airways are contraindicated or poorly tolerated. 3.Compact and Portable: NPAs occupy minimal space in trauma kits, ambulances, and field bags—ideal for rapid response teams. 2.Ready in Multiple Sizes: Most kits include a full size range (e.g., 6–9 mm ID) to suit varied patient populations, from adolescents to adults. 3.Easy to Train and Use: NPAs require minimal training compared to advanced airway tools like endotracheal tubes. EMS providers, paramedics, and even combat medics can deploy them efficiently. Conclusion Choosing the right nasopharyngeal airway involves more than just size or price. It's about understanding how material selection, design features, and clinical setting come together to ensure safe, effective airway management. For budget-conscious, temporary use, PVC-based NPAs offer practicality and reliability. For hospitals and high-care environments, silicone NPAs provide enhanced comfort, flexibility, and performance. By partnering with a reputable manufacturer like BEVER Medical that emphasizes biocompatibility, performance testing, and global regulatory compliance, you ensure your airway management solutions meet the highest clinical standards—where every second counts, and every breath matters.
View More + -
23 Jun 2025
As global demand for airway management solutions continues to rise in emergency, trauma, and tactical medical environments, disposable nasopharyngeal airway kits have become essential medical tools. Known for their ease of use, reduced infection risk, and rapid deployment, these devices are increasingly favored by healthcare providers, military medics, and OEM suppliers alike. Bever Medical shares the nasopharyngeal airway kit market's growth trends and regulations in this article. We aim to help distributors, medical device companies, and OEM customers navigate this expanding industry segment. Take a quick look now. Market and Industry Overview The disposable nasopharyngeal airway market shows strong growth potential. Market research indicates the market was valued at USD 1.5 billion in 2024 and is projected to reach USD 2.5 billion by 2033, with a compound annual growth rate (CAGR) of 6.5%. Meanwhile, the broader nasopharyngeal airway market (including reusable variants) is expected to grow from USD 250 million to USD 450 million in the same period, at a CAGR of 7.5%. Key Drivers of Market Growth Rising prevalence of respiratory emergencies and trauma cases Increased adoption of minimally invasive airway management techniques The growing elderly population requires pre-hospital care Expanding use in tactical and military rescue operations Applications in Emergency and Military Medicine Nasopharyngeal airway kits play a vital role in pre-hospital, battlefield, and disaster medicine. In tactical combat casualty care (TCCC), compromised airways are the second leading cause of potentially survivable battlefield deaths, after hemorrhage. Combat medics depend on these kits for rapid, safe airway access in high-stress scenarios. There are some advantages in field use: Suitable for unconscious or semi-conscious patients with intact gag reflexes Preferred over oropharyngeal airways in field conditions Helps facilitate bag-valve-mask ventilation Lightweight and compact for outdoor emergency kits Regulatory Compliance Requirements FDA Regulations (United States) In the U.S., the FDA classifies nasopharyngeal airways under product code BTQ, Class I medical devices (21 CFR 868.5100). These devices are 510(k) exempt, meaning no premarket notification is required. However, manufacturers must: Register their establishment Comply with Quality System Regulations (QSR) Prepare for the QMSR Final Rule (effective Feb 2, 2026), which harmonizes U.S. regulations with ISO 13485:2016 CE Marking (European Union) Under the EU MDR, nasopharyngeal airways must: Comply with ISO 13485 quality systems Meet safety and performance criteria Provide labeling per EN 1041 Ensure sterile packaging in line with EN 556-1, with a sterility assurance level (SAL) of 10⁻⁶ or lower, meaning ≤1 viable microorganism per 1,000,000 devices ISO Standards and Sterilization Requirements ISO 13485 Quality Management Systems This global standard outlines specific requirements for medical device QMS, covering: Risk management and regulatory controls Production and post-market surveillance Continuous improvement and traceability Implementing ISO 13485 signals that the manufacturer produces safe, effective devices that meet global compliance standards. Sterilization Validation For disposable nasopharyngeal airways, sterilization must be validated under: ISO 11135 (ethylene oxide) ISO 11137 (radiation sterilization) The sterilization process must ensure: Installation Qualification (IQ) Operational Qualification (OQ) Performance Qualification (PQ) All documented to confirm consistent SAL 10⁻⁶ sterility while preserving product functionality. OEM Manufacturing Considerations OEM Service Advantages OEMs offer end-to-end solutions for nasopharyngeal airway kits, including: Product design and engineering Regulatory support (FDA, CE, ISO) Scalable manufacturing and technical documentation By outsourcing to OEMs, medical brands reduce cost, shorten time to market, and shift compliance risks to trusted experts. Contract Manufacturing Benefits Contract manufacturers offer flexibility and customization, such as: Material selection and lubricant packaging Pediatric and adult size ranges Prototyping, sample validation, and batch production These services allow companies to deliver tailored products without building in-house production lines. Product Features and Selection Criteria Key Design Features Made from medical-grade PVC, soft yet structurally sound Rounded, lubricated tips for smooth insertion Flared ends prevent migration Available in multiple sizes: pediatric to adult Individually packaged and sterile Selection Guidelines Healthcare professionals should assess: Proper sizing: smaller than the patient’s nostril diameter Biocompatibility: latex-free and non-allergenic materials Flexibility: to prevent kinking during use Sterility: ensure clear labeling and expiration dates Contact Bever Medical Today Bever Medical specializes in FDA- and CE-certified disposable nasopharyngeal airway kits that meet global quality standards. Our OEM and contract manufacturing services are trusted by leading emergency medical and military organizations worldwide. Any interests, please feel free to contact us to request a quote for bulk orders or explore custom solutions for your airway management needs.
View More + -
25 Jun 2025
When it comes to airway management, the nasopharyngeal airway (NPA) is a simple yet lifesaving device. Used in both emergency and clinical settings, NPAs help maintain an open airway in semi-conscious or unconscious patients. While sizing and technique are essential, the material of the NPA can significantly impact patient safety, comfort, and overall usability. In the medical device market, NPAs are typically made from PVC (polyvinyl chloride), silicone, or latex-free compounds. Each material has its strengths and limitations. Understanding the differences is key for clinicians, purchasing managers, and distributors aiming to match product performance with patient needs and institutional goals. PVC NPAs PVC (polyvinyl chloride) is the most widely used material for disposable NPAs and many other medical products. Known for its versatility and low cost, PVC has become a go-to choice for high-volume hospital settings and emergency response kits. Key Features: Affordable and easy to mass-produce Firm enough for smooth insertion Available in multiple sizes Pros: Cost-effective: Ideal for bulk procurement and single-use applications Readily available: Compatible with most prehospital and hospital supply chains Reliable performance: Stiff but flexible when warmed or lubricated Cons: Can become rigid in cold environments, increasing patient discomfort or injury risk Not inherently latex-free — always verify if labeled “latex-free” to avoid allergy issues Best for: EMS kits, military medical supplies Budget-sensitive facilities Disposable product use where patient interaction is brief Silicone NPAs Silicone NPAs are considered a premium option due to their high biocompatibility and soft, flexible structure. They are often used in settings where patient comfort is a top priority, including pediatric and geriatric care or when multiple airway insertions may be needed. Key Features: Inherently hypoallergenic Very soft and flexible, even in cold temperatures Can be sterilized or reused in select settings Pros: Superior patient comfort: Reduces risk of mucosal injury or discomfort Excellent for long-term or conscious use: Less likely to cause gag reflex Flexible in all environments: Ideal for transport or variable-temperature settings Cons: Higher cost: May not be feasible for single-use, high-volume settings Can be slippery to handle: Requires careful insertion and handling when lubricated Best for: Pediatric, elderly, or conscious patients Hospitals focused on comfort and care quality Facilities able to accommodate higher per-unit cost Latex-Free NPAs “Latex-free” refers to a product’s absence of natural rubber latex, a known allergen that can trigger severe reactions in sensitive patients and healthcare workers. Latex-free NPAs may be made from PVC, silicone, or thermoplastic elastomers (TPE). Key Features: Allergy-safe for patients and staff Increasingly required by regulatory standards Can be made from various materials Pros: Essential for modern healthcare settings: Helps meet hospital safety policies Widely accepted internationally: Compliant with CE, FDA, and ISO regulations Flexible base options: Can still benefit from PVC or silicone properties, depending on product Cons: Performance varies by base material: “Latex-free” is a safety label, not a performance indicator May be more expensive than non-certified alternatives Best for: General hospital use Pediatric and allergy-prone patients International buyers with latex-free compliance needs Quick Comparison Table Feature PVC Silicone Latex-Free (Varies) Cost Low High Varies Flexibility Medium High Depends on material Patient Comfort Moderate (with lube) Excellent Varies Allergy Risk Check label Very Low None Best Use Emergency, EMS Comfort care, ICU Hospitals, Export Conclusion Choosing the right NPA material is about more than price—it's about matching patient needs with the right product features. PVC offers excellent value for one-time use in high-volume or emergency scenarios. Silicone excels in sensitive patient groups and long-term care. Latex-free is no longer optional—it’s a minimum standard in most modern healthcare systems. Whether you are sourcing for a hospital, clinic, or EMS provider, always ensure material details are clearly stated on packaging and supported by quality certifications (e.g., CE, FDA, ISO 13485). Call to Action Looking for reliable, high-quality nasopharyngeal airways in PVC, silicone, or latex-free options?BEVER Medical offers a full range of NPAs in all adult and pediatric sizes, ready for OEM, bulk purchasing, and international delivery.Contact our sales team today to request product samples, specification sheets, or custom branding options.
View More +



 English
English